A molecule of fat is three long chains of carbon atoms and hydrogen atoms connected to a head. The heads aren't important to this discussion. The tails look like this:
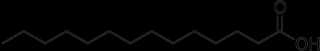
(sorry about the pictures--I don't know why they are black. Click 'em, and you can see what I'm talking about)
Each line represents a bond between two carbon atoms. Carbon forms four bonds; in this picture, the carbons are each bonded to two other carbons, and two hydrogens. This fat is saturated--each carbon has a full compliment of hydrogens. These long, straight chains pack easily, so saturated fats are solid at room terperature. They are mainly found in animals--think butter, lard, and bacon.
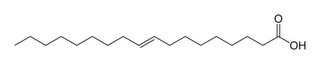
This is an unsaturated fat. Notice the double bond beween two carbons that kinks the chain. These fats don't pack well, so they are liquid at room temperature. Think olive oil, corn oil, and vegetable oil.

Unsaturated fats have a problem, though. That extra bond is just itching to break and pick up something else, like oxygen. Especially when it gets hot. The oxygen--or other stuff--makes the food cooked in the fat taste bad. This isn't a problem in a home kitchen, because the fat doesn't stay hot long, and the fat rarely gets used repeatedly. In a commercial fryer, though, the fat hangs around for a while, and that can be a problem. The solution is hydrogenation--a process where the double bonds get broken and filled with hydrogen. This makes the fat chemically similar to animal fats (but to most people, "partially hydrogenated soybean oil" sounds better than "lard" or "tallow.")
That gives us this:
This is a trans fat. It's still unsaturated, but it's straight. That double bond actually makes it stiffer, and easier to pack. Trans fats are solid at room temperature. Think vegetable shortening. Trans fats are a the fat that is most responsible for heart disease, and are also a carcinogen.
It turns out that butter is better than margerine, and lard is better than shortening. The old ways are still the best.
(Nerd note: trans fats are called that because of the arrangement of the hydrogens around the bond. They are across the chain from each other--trans in latin. The bent unsaturated fats are cis unsaturated, because the hydrogens are on the same side of the bond.)

No comments:
Post a Comment